Phytopharm is seeking to strengthen its partnering package for its Parkinson’s disease candidate Cogane with the start of an important Phase I bioavailability study with solid dose capsule formulations. Results by end 2012/early 2013 are significant to making Cogane, currently being tested as a slightly less convenient liquid formulation, more commercially viable and attractive to potential partners. However, Cogane’s true magnetism is ultimately dependent on top-line results in February 2013 from the Confident-PD Phase II study, a binary event for Phytopharm. We maintain our rNPV of £48m while noting that success in both trials and securing a partner would raise our valuation to £83m.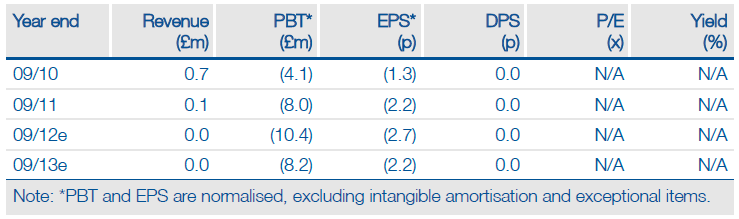
Getting Phase III ready
Cogane has the potential to become the first small-molecule, disease-modifying therapy for Parkinson’s, and therefore could attract a lucrative licensing deal. However, this depends on the outcome of the Confident-PD study in 408 patients with newly diagnosed, treatment-naive Parkinson’s disease, which should render top-line results in February 2013. The concurrent development of a solid dose capsule formulation of Cogane is also important as positive results in both studies would present potential partners with a Phase III-ready asset and help Phytopharm extract best value from partnership discussions. We recently published full details of the Confident-PD study, Phytopharm’s other pipeline projects and the overall investment case.
ALS option
Cogane has also demonstrated potential as a treatment for amyotrophic lateral sclerosis (ALS), with positive data from four different models of ALS. Depending on the outcome of Confident-PD, ALS could offer an additional valuation parameter in any licensing discussions or may provide a fall-back avenue of development for Cogane if the drug fails in the Parkinson’s trial.
Valuation: rNPV of £48m with significant uplift potential
We maintain our risk-adjusted NPV of £48m, which compares favourably with Phytopharm’s £10.4m enterprise value, based on net cash of £13.3m as of 31 March 2012. Over 50% of our valuation is assigned to Cogane’s potential in Parkinson’s. Ahead of data from the Confident-PD trial, we assign a 25% probability of success – positive results and securing a partner would raise that probability to 50% and therefore our rNPV to £83m. Phytopharm has reiterated prior guidance that existing cash reserves are sufficient to fund operations to the end of 2013.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Phytopharm IMS: Cogane Solid Dose Development
Published 08/17/2012, 07:07 AM
Updated 07/09/2023, 06:31 AM
Phytopharm IMS: Cogane Solid Dose Development
Commercial formulation required
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.