Shares of Alnylam Pharmaceuticals Inc. (NASDAQ:ALNY) were up 10.5% after the FDA approved its givosiran injection for subcutaneous use for the treatment of adults with acute hepatic porphyria (AHP). The approval came in three months before the PDUFA date of Feb 20, 2020. Gvosiran injection will be marketed by the trade name of Givlaari. This is the second RNAi therapeutic from Alnylam to have received an FDA approval in the last 16 months and the first-ever galnac-conjugate RNA therapeutic to get an approval, marking a significant advancement of precision genetic medicines. Givlaari is expected to be available in the United States by the end of 2019. Reportedly, the drug is priced considerably high at $575,000 per annum.
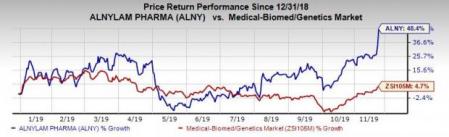
Shares of the company have surged 48.4% year to date compared with the industry’s growth of 4.7%.
Givlaari’s approval was based on the positive results from the phase III ENVISION study, which showed that AHP patients treated with this drug experienced 70% lesser porphyria attacks compared to placebo. Givlaari also led to a similar reduction in intravenous hemin use, as well as reductions in urinary aminolevulinic acid (ALA) and urinary porphobilinogen (PBG) compared to placebo.
Givlaari was reviewed by the FDA on a priority review basis and had previously been granted Breakthrough Therapy and Orphan Drug designations in the United States. The drug is currently being reviewed under accelerated assessment by the European Medicines Agency (EMA).
The company also announced that it reached value-based agreements (VBAs) in principle with Harvard Pilgrim Healthcare to cover the drug. The framework is designed to accelerate patient and provider access to Givlaari. Under this agreement, Alnylam would be paid the drug’s full price based on the ability of the same to deliver outcomes in the real world setting compared to those seen in clinical trials.
Currently, the population of AHP patients diagnosed with active disease in the United States and Europe is estimated to be about 3,000.
Last year, the company received approval from the FDA for Onpattro lipid complex injection, a first-of-its-kind RNAi therapeutic, for the treatment of the polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults.
Another drug from Alnylam’s pipeline, which could be filed soon with the FDA, is inclisiran that is being developed to treat hypercholesterolemia in partnership with The Medicine Company (NASDAQ:MDCO) . The companies have completed most of the phase III ORION studies on inclisiran and plan to submit an NDA with the FDA by this year.
Zacks Rank & Stocks to Consider
Alnylam currently has a Zacks Rank #3 (Hold).
A few better-ranked stocks in the biotech sector are Alkermes Plc. (NASDAQ:ALKS) and Anika Therapeutics Inc. (NASDAQ:ANIK) , both sporting a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Alkermes’ earnings per share estimates have increased from 36 cents to 52 cents for 2019 in the past 60 days. The company delivered a positive earnings surprise in the trailing four quarters by 236.80%, on average.
Anika’s earnings per share estimates have increased from $1.75 to $2.03 for 2019 and from $1.36 to $1.62 for 2020 in the past 60 days. The company delivered a positive earnings surprise in the trailing four quarters by 53.31%, on average.
Just Released: Zacks’ 7 Best Stocks for Today
Experts extracted 7 stocks from the list of 220 Zacks Rank #1 Strong Buys that has beaten the market more than 2X over with a stunning average gain of +24.5% per year. These 7 were selected because of their superior potential for immediate breakout.
See these time-sensitive tickers now >>
The Medicines Company (MDCO): Free Stock Analysis Report
Anika Therapeutics Inc. (ANIK): Free Stock Analysis Report
Alnylam Pharmaceuticals, Inc. (ALNY): Free Stock Analysis Report
Alkermes plc (ALKS): Free Stock Analysis Report
Original post
Zacks Investment Research